In a groundbreaking new study, researchers from the Wellcome Sanger Institute, along with collaborators from the University of Exeter and the University of Cambridge, have made significant strides in simplifying the process of diagnosing rare developmental disorders in children. By reassessing genetic data from nearly 10,000 families, they have demonstrated that a single genetic test, specifically exome sequencing, can be as accurate, if not better, than the current two-step approach using standard microarrays. This shift has the potential to offer earlier diagnoses for families and save valuable resources for the NHS.
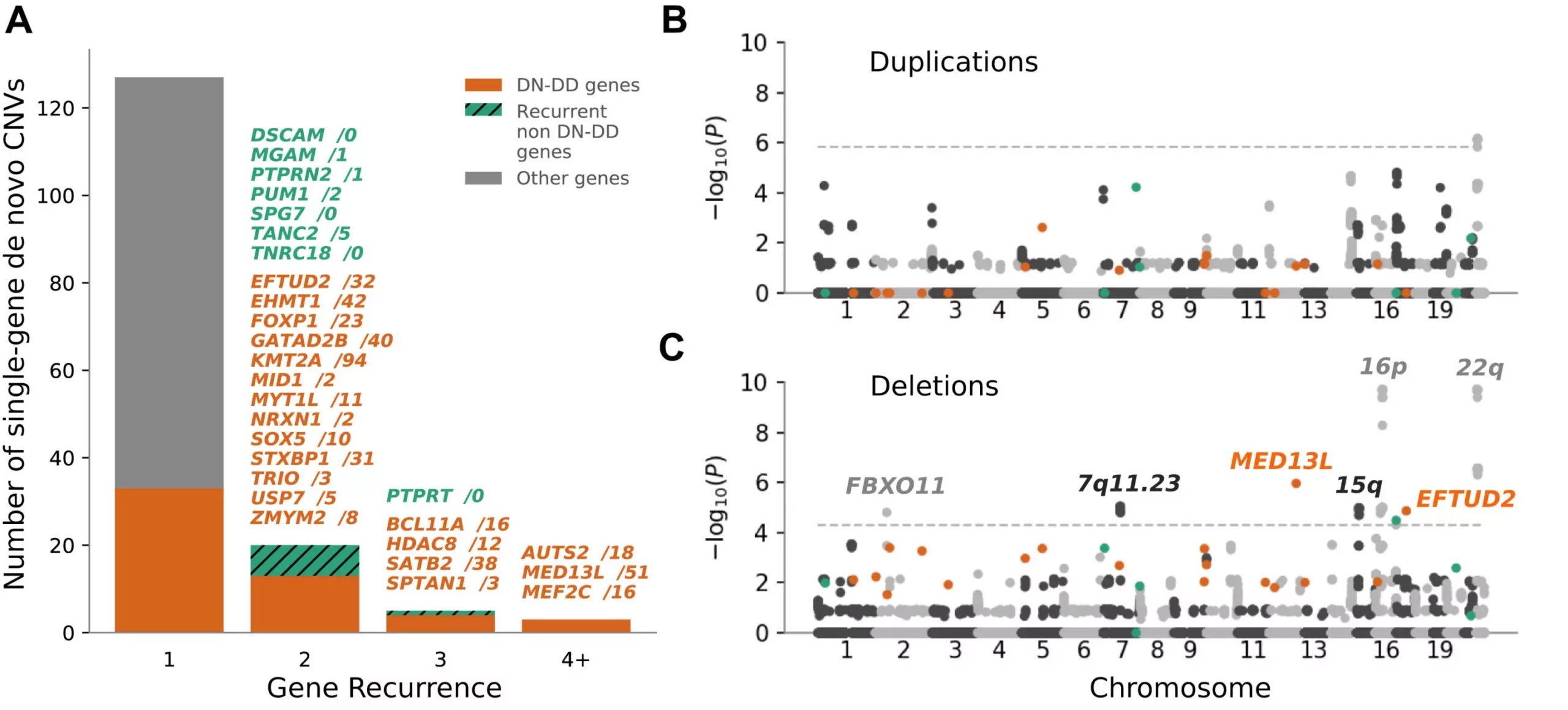
Genetic changes can vary from single letter alterations to larger structural variations in DNA known as copy number variations (CNVs). These CNVs can sometimes lead to neurodevelopmental disorders in children, such as Angelman syndrome, DiGeorge syndrome, and Williams-Beuren syndrome. Detecting these large-scale variations can be challenging for clinical teams, which is why microarrays have been traditionally used in the diagnostic process. However, these tests often require subsequent genome-wide sequencing for a comprehensive analysis, leading to a lengthy and costly process for families.
The researchers set out to streamline the diagnostic process by developing a single-assay approach using data from exome sequencing. By combining four algorithms and utilizing machine learning techniques, they were able to detect 305 large-scale pathogenic mutations, including 91 that were previously undetectable with standard microarrays. This new approach not only simplifies the testing process but also eliminates the need for multiple tests, providing a comprehensive analysis in a single test.
Professor Caroline Wright from the University of Exeter emphasizes the significance of this breakthrough, stating that it marks a significant step forward in making genetic testing more accessible and cost-effective. The traditional step-wise process of multiple genetic tests may soon be replaced by a single test, offering hope for faster and more accurate diagnoses for families. Professor Helen Firth from the University of Cambridge echoes this sentiment, highlighting the potential for families to receive a diagnosis more efficiently in the future.
As our understanding of genetic variations continues to evolve, the adoption of this new single-assay approach in clinical practice is crucial. Professor Matthew Hurles, director of the Wellcome Sanger Institute, underscores the importance of providing adequate bioinformatics training to support the widespread use of this new testing method. With the right computational tools and training, genetic testing can become simpler, cheaper, and more accessible, ultimately benefiting both patients and healthcare systems.
The groundbreaking study on utilizing exome sequencing for diagnosing rare developmental disorders represents a significant advancement in genetic testing. By streamlining the diagnostic process and improving the accuracy of identifying structural genetic variations, this new approach has the potential to revolutionize how we diagnose and treat genetic diseases. As researchers continue to refine and expand upon these findings, the future of genetic testing looks promising for both patients and healthcare providers.